Product Serialization: Benefits, Process & Compliance
Learn how product serialization boosts compliance, security, and traceability in supply chain with benefits, processes, and why it matters for global trade.
200+ buyers trust Torg for sourcing

Walk into a pharmacy or pick up a gadget in an electronics store, and you’ll notice the packaging looks simple enough. But behind every box, bottle, or device is a hidden safeguard: product serialization. Think of it as giving each item its own digital passport.
In the pharmaceutical sector, literally lives can be saved. But it can also be the same with food, cosmetics, or even high-end merchandise, serialization ties all steps of the entire supply chain, from raw materials to the end user, in a secured and regulated way.
Businesses gain as well. With a distinct identifier on each product unit, supply chain partners handle trace and authenticate products, allowing for faster and safer quality control, product recall, and regulatory compliance. This article demystifies the process of serialization, its main advantages, and why it is transforming global supply chain transparency.
What Is Product Serialization?
Product serialization is the process of assigning a unique identifier (such as a serial number, barcode, or QR code) to each individual product or package. Unlike batch or lot numbers, serialization provides item-level traceability, meaning every single unit can be tracked throughout the entire supply chain—from manufacturing and distribution to retail and even post-sale verification.
Consider the pharmaceutical industry, where safety is very important. Every package of medication has to carry an individualized identifier. This allows pharmacists, regulators, and even the end user to verify authenticity in a controlled and secure manner. The same principle can be applied to electronics, beauty products, food items, or any industry where trust and quality control is key.
The true objective? To trace and track products through the entire supply chain. From raw material sourcing to the end product, serialization makes every step transparent, and auditable. Companies can identify the specific units that are affected if something goes wrong (e.g. a food contamination or a bad batch of parts) rather than having to recall everything. That's the strength of assigning each product its own individual identity.
Serialization vs. Barcoding vs. Batch Numbering

Batch numbers, barcodes, and serialization may look the same at first sight. Indeed, they all are codes printed on packages. But when you look closer, each one of them is actually used for a very different purpose in how goods flow through the whole supply chain.
Let's begin with batch numbering. Imagine a bakery that makes 5,000 loaves of bread in one morning. All the loaves will have the same batch number since they were all produced in the same conditions. If there is a fault (e.g., the flour was faulty) the bakery can do a product recall on the entire batch. It's handy, but it isn't useful if you need to identify a specific loaf.
Barcoding goes one step ahead. Each SKU has its own barcode which the retailers use to process sales and inventory. For example, two bottles of the same brand and size shampoo will have the same barcode. But that’s where the trick comes in: barcodes can’t tell the difference between those two bottles. If one was the original and the other fake, the barcode would not be able to tell the difference.
This is where serialization really makes a difference. Throughout the serialization process, every unit of product is assigned an individual serial number or unique code (most likely a QR code or RFID tag) that links back to a master database. Consider two looking-alike boxes of medication. Serialization allows them to each have separate identifiers so that it can be traced and tracked in a secure and controlled way. This gives supply chain visibility, quality control, and makes counterfeiting much easier to detect.
In short, batch numbers aggregate products, barcodes mark product types, but serialization assigns each individual item a unique identity. And that’s what keeps today’s supply chains secure and efficient.
Benefits of Product Serialization
Before serialization, businesses had no way to track their products all the way through the supply chain. Now, assigning a unique number to every product unit makes it transparent, safe, and gives brands and customers more confidence in what they are working with. Let’s look at the key benefits in more detail.
Fighting Counterfeit Products
Counterfeits drain billions from the global economy and pharma is one of the most affected. By putting serial numbers or QR codes in product packaging, businesses can verify products in an authoritative and secure way. For example, a patient can scan a pack of medicine and verify it’s authentic in seconds. For businesses, that means lower liability, less risk, and more brand confidence.
Enhancing Product Traceability
Serialization allows supply chain traceability that older systems couldn't achieve. Each product can be traced from raw materials to the final product on the shelf. Should a contamination or flaw develop, serialization enables targeted recalls of the product, isolating individual units rather than pulling entire lines. This not only helps protect customers but also saves companies money and reputation damage.
Strengthening Regulatory Compliance
Serialization is not optional. Governments everywhere don’t leave serialization to choice, they insist. Regulations like the DSCSA in the US or the EU FMD in the EU require pharmaceutical products to be tracked and authenticated in a controlled and secure environment. By providing individual serial numbers and keeping accurate records in a centralized database, companies show the regulators that each product unit is safe to sell. You can’t bypass compliance. It will result in frozen shipments, big fines, or even loss of access to key markets. So serialization is both a tool for label compliance and protection from legal exposure.
Enhancing Operational Efficiency
Serialization may initially seem like more paperwork, but with automated systems, it eliminates inefficiencies. Consider a warehouse manager scanning hundreds of boxes. Without serialization, bugs enter like incorrect items shipped, mislabeled packages, or forgotten batch numbers. With serialization, each scan re-ties back to one singular identifier so that quality control is easier and logistics quicker.
Improving Supply Chain Visibility
Serialization builds trust among supply chain partners. As goods move from manufacturer to distributor to retailer, each step can be verified in a secure environment. This supply chain visibility means fewer disputes, faster issue resolution, and products were warehoused and shipped correctly.
Establishing Consumer Confidence
Lastly, consider the final consumer. A parent purchasing infant formula, or an investor who's purchasing high-end cosmetics, needs assurance. When they scan a QR code or check a unique identifier, they know the product is genuine. That moment of trust is more than safety. It generates long-term loyalty. In intensely competitive markets, customers are more likely to remain loyal to brands that can demonstrate authenticity and supply chain traceability on the packaging.
Regulatory Compliance
Serialization is the rule in most locations. Serialization is the norm. Governments around the world have recognized that counterfeit drugs and untraceable goods kill, especially in the pharma industry. That’s why they have introduced strict regulations to ensure pharma products and other goods move through the entire supply chain in a controlled and safe way.
DSCSA, EU FMD, and Other Compliance Regulations
Consider the case of DSCSA (Drug Supply Chain Security Act) in the U.S. It mandates that each prescription medication must have a unique identifier so that each unit of a product can be authenticated. The intention is patent: improve supply chain visibility and safeguard patients.
In Europe, even the EU FMD (Falsified Medicines Directive) takes it a step further by requiring tamper-evident packaging in addition to serialization. That way, if the package has been opened or tampered with, it's clearly apparent.
And it's not merely the U.S. and Europe. Brazil, India, Russia, and China have launched their own schemes. The message is one for all: you can't ensure authenticity without serialization.
Serialization Levels: Primary to Pallet
One of the most fascinating things about the serialization process is that it doesn't merely end with one bottle or box. It is done in layers, touching every step of packaging.
- Primary packaging: This might be the pill bottle itself, each carrying a unique serial number or QR code.
- Secondary packaging: A box containing multiple bottles, connected digitally with the codes within.
- Tertiary packaging: Stacked big cartons on a pallet, containing aggregated information that ties together all the individual parts within.
This tiered system is more traceable. If one batch gets contaminated, businesses don't have to second-guess, they can identify the specific batch number or unit. It's akin to having a family tree for each shipment, where it clearly displays where each product originated from and where it is going.
How Does the Serialization Process Work?
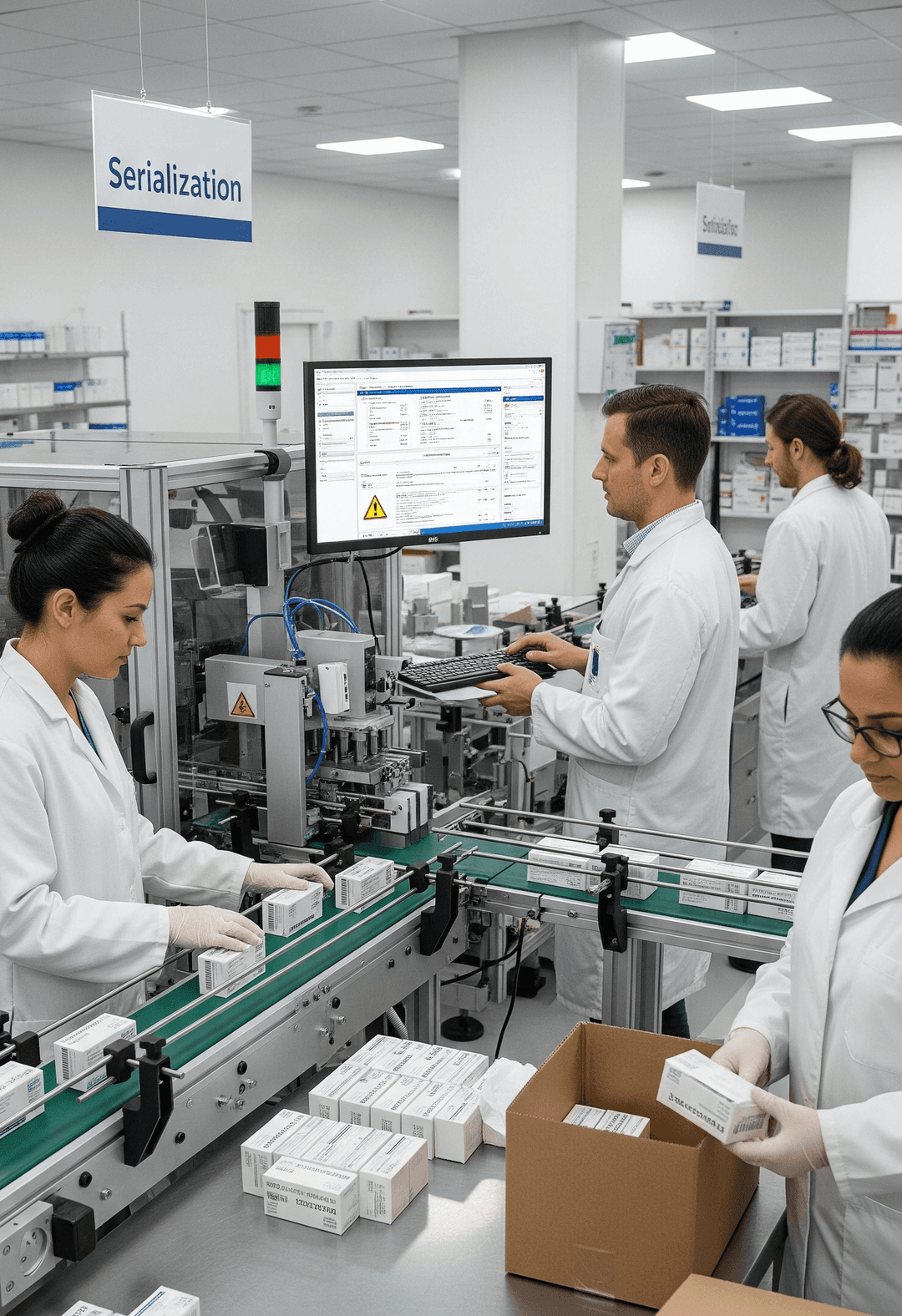
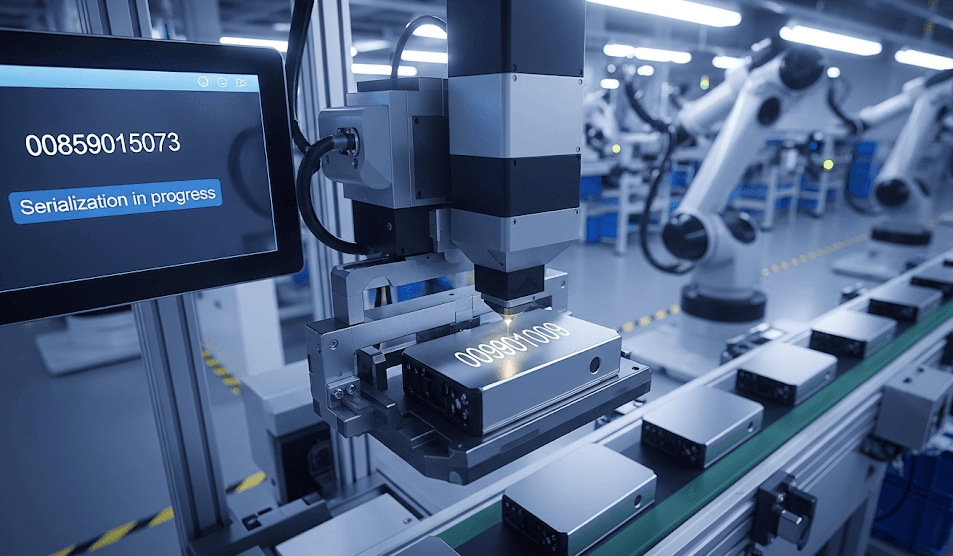
So you ever wondered how a single product gets its own digital fingerprint? It’s in the serialization process. Sounds complicated but when broken down it’s a simple sequence of automated systems that connect manufacturers, distributors, and other supply chain partners.
- Generating Serial Numbers: The initial process is creating serial numbers. They're not random numbers—they're specific identifiers created to make sure that no two products within all product lines overlap. It's like issuing every product its own passport, a document of identification that accompanies it throughout the entire supply chain.
- Assigning Numbers: Once they're generated, these identifiers are linked to products in a secure way. They may be in the form of an imprinted serial number, an easily visible QR code, or an RFID tag inserted into packaging. Each method allows the end customer or business to check for authenticity at the touch of an instant.
- Data Capture: This is where it gets strong. Each identifier is linked to information such as the batch number, stock keeping unit (SKU), and end product description. This information is stored in a master database, providing a permanent record that can be accessed by approved parties.
- Aggregation: Serialization isn't just about the smallest unit. Individual codes are digitally attached to larger packaging units such as cartons containing multiple bottles, or a pallet loaded with cartons. This facilitates easier tracing of individual components back to their point of origin without opening every box.
- Verification: Lastly, as products travel through the supply chain, distributors scan codes to verify authenticity. Retailers check inventory, distributors inspect shipments, and consumers are able to scan to ensure safety all in a secure and controlled fashion.
This whole process builds a living digital map of all serialized products. From raw materials to the end product in the hands of the customer, businesses have unparalleled supply chain visibility, and counterfeiters have fewer hideouts.
Key Industries Using Product Serialization
Serialization is not specific to one industry. It’s across the board in industries where safety, trust, and authenticity matter. Whether it’s a medicine that could save a life, a piece of chocolate, or a designer handbag, assigning a unique identifier to every product unit makes the whole supply chain more trustworthy.
Pharmaceuticals & Life Sciences
No sector relies on serialization as heavily as the pharmaceutical sector. Each box of medicine, bottle, or vial has a special serial number traceable back to a central database. It prevents counterfeiting, allows for product recall to be extremely targeted, and most importantly, safeguards patients from fraudulent drugs. Think of a pharmacy scanning an item and immediately verifying the end product is authentic, that sense of security saves lives.
Food and Beverage
In the world of food, supply chain traceability can be the difference between a rapid response and a PR nightmare. Should there be contamination, serialization allows problems to be traced to raw materials or a batch number. Rather than recall all products on store shelves, businesses can recall just the units in question, minimizing waste and brand and consumer protection.
Consumer Electronics
Fake smartphones, tablets, gadgets, and new technologies overwhelm markets globally. By assigning an individual identity to each device, sometimes through a QR code or RFID tag—companies can combat fraud and theft while providing more seamless warranty and after-sales assistance. For example, a user who registers a phone's unique code guarantees the device as genuine and serviceable.
Cosmetics & Luxury Goods
Where luxury is concerned, reputation is paramount. Value-added serial products such as designer fragrances or high-end skin care feature easily readable identifiers that consumers can scan to verify on the spot. Aside from eliminating counterfeit goods, this creates greater loyalty. A consumer who believes a product is authentic is much more likely to remain loyal to the brand in the long run.
Global Serialization Regulations and Standards
Various governments have formulated their own regulations to ensure products that are serialized flow all the way through the supply chain in an orderly and secure way. The challenge for international companies is that these regulations don't necessarily appear the same from one border to another. This is why knowledge of local law and international guidelines is critical.
United States
Within the U.S., the Drug Supply Chain Security Act (DSCSA) makes each package of prescription medication carry a unique identifier. The legislation has strict milestones, culminating in full track and trace implementation throughout the supply chain. By the end, each and every unit of medicine can be authenticated and traced back to its origin.
European Union
Europe is very serious about serialization with the Falsified Medicines Directive (EU FMD). Not only does it require serial numbers that are unique, but it also calls for tamper-evident packaging. This manner, supply chain partners and the end consumer can easily verify if a product has been tampered with, safeguarding patients against counterfeit products.
China, Brazil, Russia, India
Others are following suit too. China, Brazil, Russia, and India have enacted their own product serialization regulations. Although the requirements differ such as the reporting of serial numbers, or the reporting of data, the underlying theme is accountability. All the laws encourage businesses to implement a serialization process that enhances supply chain traceability and ensures better quality control.
GS1 Standards for Serialization
Because every region has its own regulations, the international market depends on GS1 standards to maintain uniformity. GS1 standards determine how a distinct code, QR code, or RFID tag must be created, stored in a global database, and disseminated throughout borders. In adhering to GS1, businesses ensure that information moves continuously between all of the supply chain partners managed globally. Without this uniformity, international distribution would be a patchwork quilt of incompatible systems.
Serialization vs. Track and Trace
Serialization and track and trace tend to be discussed side by side, but they are not the same. They're two sides of the same coin.
Serialization is the process of giving a distinct identifier such as a serial number, QR code, or RFID tag to each individual unit. It's what provides a product with its digital fingerprint. Two identical packets of medicine, for instance, may appear the same on the outside, but serialization means that each one has its own individual code embedded in a central database. This is the building block on which all else is established.
Track and trace, however, is what takes place after that fingerprint is created. As the product travels through the whole supply chain, from manufacturer to distributor to retailer to the eventual end consumer, its identity is read, captured, and confirmed. In reality, it's like stamping that product's passport at every border.
Combined, serialization and track and trace provide supply chain visibility in a secure and controlled way. They enable quality control, product recall, and supply chain traceability to be more simple and quick. If ever you have an issue (say, a faulty specific component or a batch that must be recalled) you don't have to look around blindly. The information is revealing you precisely where every unit of a product went, to whom, and how to return it.
Conclusion
Serialization is one of the biggest tools available for supply chain management today, especially in the pharma industry. By assigning a unique digital “fingerprint” to each item, businesses can ensure authenticity, safety, and compliance.
It’s not just about preventing counterfeits. Serialization also enables faster recalls, builds trust between supply chain partners, and keeps operations running. As global regulations and GS1 guidelines push the practice forward, serialization is now the foundation of traceability.
In the future, more industries will adopt serialization as a way to put transparency and safety at the centre, making supply chains smarter, and more secure for all.
FAQs
1. What does it mean for a product to be serialized?
When a product is serialized, it gets a one-off identifier like a QR code, RFID tag, or serial number that’s its digital fingerprint. The code makes the product trackable throughout the entire supply chain from raw materials to the end customer.
2. What is the purpose of serialization?
The main purpose is traceability across the supply chain. Serialization prevents counterfeits, enables companies to do rapid product recalls, complies with regulations, and ensures products are handled in controlled and secure conditions. In short, it’s about brand protection and consumer protection.
3. What is the process of serialization?
The serialization process starts by generating serial numbers to ensure uniqueness. They are applied to every unit of product with a unique code (barcode, QR, or RFID). The information (batch number, SKU, and end product data) is stored in a single database. Supply chain partners can scan and authenticate products from there and product tracking becomes seamless.
Request a Bulk Order Quote
Simple ordering, transparent pricing, delivered straight to your door

